Processing Function
The Common Vein Copyright 2010
Introduction
The processing occurs in the apical membrane of the follicular cells once the iodine has been transported up to that place.
Processing Function |
|
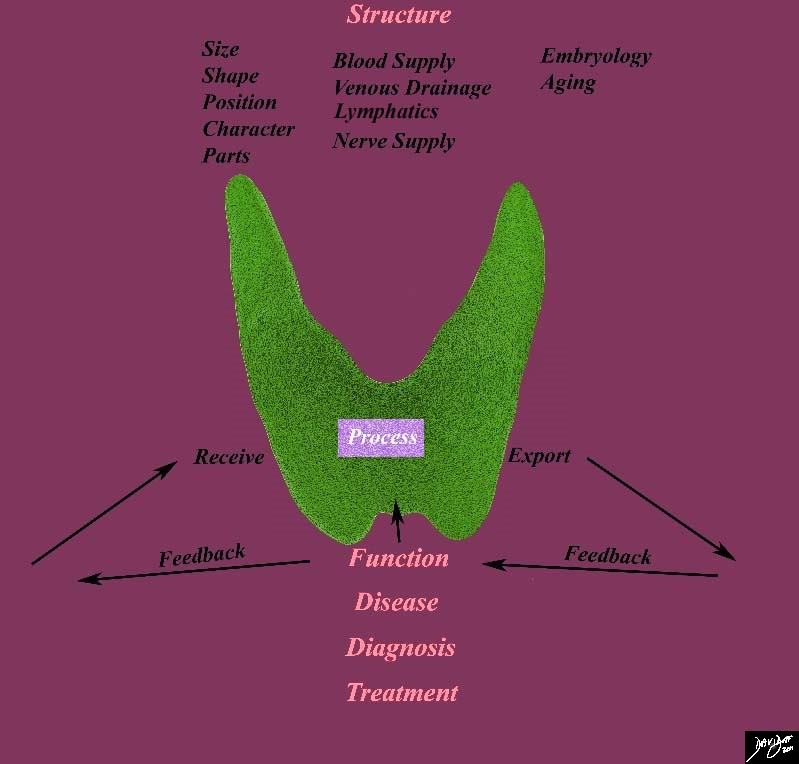
This diagram frames the underlying principles and approach to the thyroid in this module and focuses on the processing function of the thyroid Courtesy Ashley Davidoff MD 93852g05.8sb02c |
There are two major reactions,that involves thyroid peroxidase and hydrogen peroxide in order to oxide iodine. Once iodine gets oxidized, the reactive iodine atom is added to tyrosyl residues within the Tiroglobulin (Tg) to form iodotyrosines. Also called colloid, Tg is a large dimeric protein with a specific molecular weight of 660 kDa and compound by 2769 aminoacids. In the Tg the iodotyrosines are coupled using an ether linkage, this reaction is catalyzed by the Thyroid peroxidase; depending on the number of iodine molecules added to each iodotyrosine the final result is either T3 or T4. After finishing the coupling face, Tg is transported back into the follicular cell specifically into the lisosomes to finish processing the T4 and Te and than release them into the bloodstream. There is a recycling process that also occurs in order to preserver the iodine that is not coupled, therefore, mono and diiodotyrosines (MIT, DIT), are deiodinated by the enzyme dehalogenase.
Any of these reactions can be affected by an enzyme mutation leading to a defect in the synthesis of thyroid hormones and therefore developing congenital hypothyroidism, but overall, these are rare and the majority are due to recessive mutations in the thyroid peroxidase ot Tg. The clinical scenario would be an association between high levels of TSH in the setting of a pt with a large goiter.
TSH regulates thyroid gland function through two main pathways. Binding the TSH-R (a 7-transmembrane G protein-coupled receptor), TSH coupled to the a-subunit located in the Gprotein, activating a cascade where adenilcyclase is stimulated and increases direct production of cyclic AMP. The other pathway is through the direct stimulation of Phospholipase C leading to the activation of phosphatidylinositol.
Mutations of the TSH-R gene lead to different types of decrease or increase thyroid function in a congenital manner and most of them are located in the transmembrane domain of the receptor. In recessive loss-of-function mutations a hypoplastic thyroid is characteristic and evidence by congenital hypothyroidism, in the other hand Dominant gain-of-function mutations can be seen in as familial hyperthyroidism, evidence by goiter with thyroid cell hyperplasia and autonomous function of the gland.
There are growth factors that regulate thyroid gland hormone synthesis and release although TSH is dominant one. Most of them are produced in the thyroid gland such as insulin-like growth factor I (IGF-I), epidermal growth factor by transforming growth factor b (TGF-b), endothelins and different types of cytokines. The way they lead their actions and the importants of each of them individually are still in study but they are definitely involve in particular thyroid diseases. To illustrate this matter it has been described cytokines and interleukins production in association with autoimmune thyroid disease where some of them induce thyroid growth whereas others induce cell apoptosis. Another example is seeing in acromegaly where characteristic multinodular goiter is induced by increase IGF-I and growth hormone factor.